12+ orbital diagram of silver
53 from Chapter six is asking us to use orbital diagrams to describe the electron configuration of the Vaillant Shell for five different atoms. 12 1 0 11 12 11 11 55 4d Write the quantum numbers that describe the circled electron.
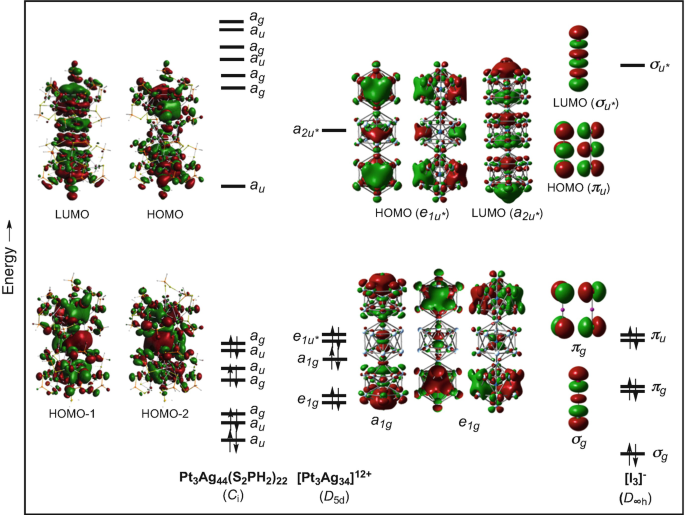
Electron Counting In Ligated High Nuclearity Late Transition Metal Clusters Springerlink
The order of filling the orbitals with electrons in.

. We use the Aufbau process to write the electronic distribution of the. PR3 11n obtained from the reaction of silverI salts with sterically. The orbitals are d xy d yz d zx d x2-y2 and d z2 and each orbital can have a maximum of two electrons.
The electron configuration of silver is 1s2 2s2 2p6 3s2 3p6 4s2 3d10. Let me explain the exact meaning of this. Yes Silver is a transition metal because it has incompletely filled d-orbital in its common oxidation state Ag 2.
1049 gcm 3. A quick glance at the Periodic Table tells us that ZAg 47 and so for the NEUTRAL atom. Orbital Diagram of All Elements.
Located in the V period. The number of electrons in the atom is. So the electron configuration of chlorine Cl in an excited state will be 1s 2 2s 2 2p.
There are different types of orbitals that all have different energy. Orbital diagram of Magnesium Mg 13. 1s 2s.
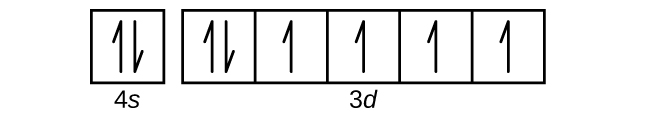
Orbital diagram of Aluminum Al 14. Ag Silver is an element with position number 47 in the periodic table. Orbital diagram silver.
Orbital diagram of Phosphorus P 16. Atomic orbital diagram of calcium 2 ion. The orbital diagram will be filled in the same order as described by the Aufbau principle.
12 The following atomic orbital diagram represents the valence electrons for silver Ag. The electron configuration of an atom is 1s 2 2s 2 2p 6. Orbital diagram of Silicon Si 15.
Syntheses spectroscopy and single crystal X-ray structural characterizations are recorded for a number of adducts AgX. 12 orbital diagram of silver Minggu 23 Oktober 2022 The electron configuration of an atom is 1s 2 2s 2 2p 6. The order in which the orbitals are filled with electrons from lower energy to higher energy is.
Electron orbital nitrogen configurations mishkanet. The electron configuration of silver is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d10 or Kr 5s1 4d10.
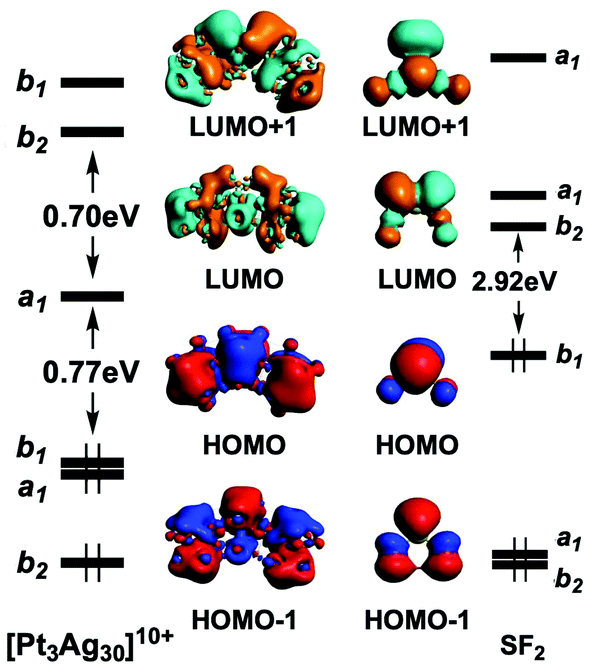
Electron Counting And Bonding Patterns In Assemblies Of Three And More Silver Rich Superatoms Nanoscale Rsc Publishing Doi 10 1039 D0nr05179a

Superatomic Three Center Bond In A Tri Icosahedral Au36ag2 Sr 18 Cluster Analogue Of 3c 2e Bond In Molecules The Journal Of Physical Chemistry Letters
Electron Configurations Orbital Box Notation M7q7 Uw Madison Chemistry 103 104 Resource Book

Unit 7 Quantum Theory Electron Configuration And Periodicity Ppt Download
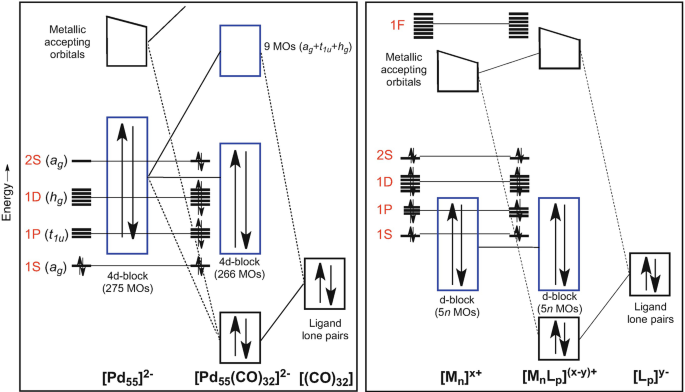
Electron Counting In Ligated High Nuclearity Late Transition Metal Clusters Springerlink
M7q7 Electron Configurations Orbital Box Notation Chem 103 104 Resource Book

Silver Ag

Endohedrally Doped Cage Clusters Chemical Reviews

Polyhydrido Copper Nanoclusters With A Hollow Icosahedral Core Cu30h18 E2p Or 2 12 E S Or Se R Npr Ipr Or Ibu Barik 2020 Chemistry 8211 A European Journal Wiley Online Library
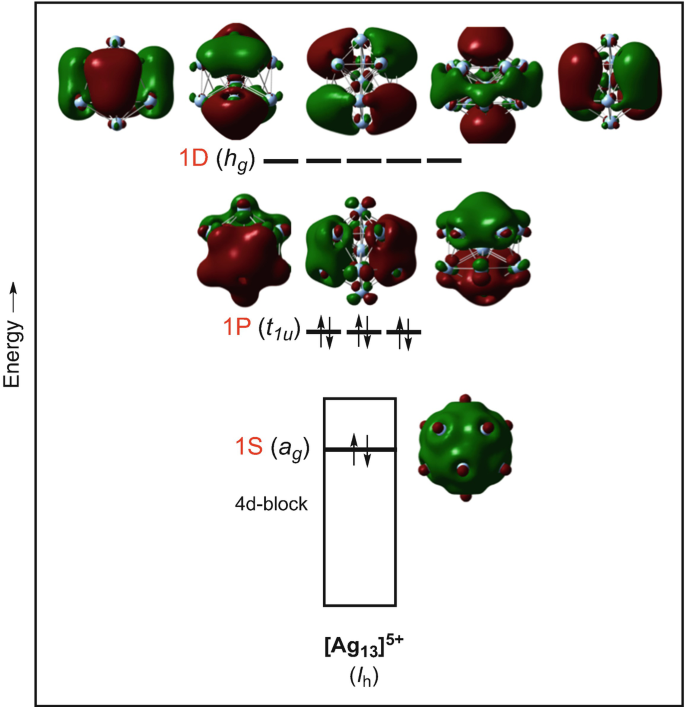
Electron Counting In Ligated High Nuclearity Late Transition Metal Clusters Springerlink
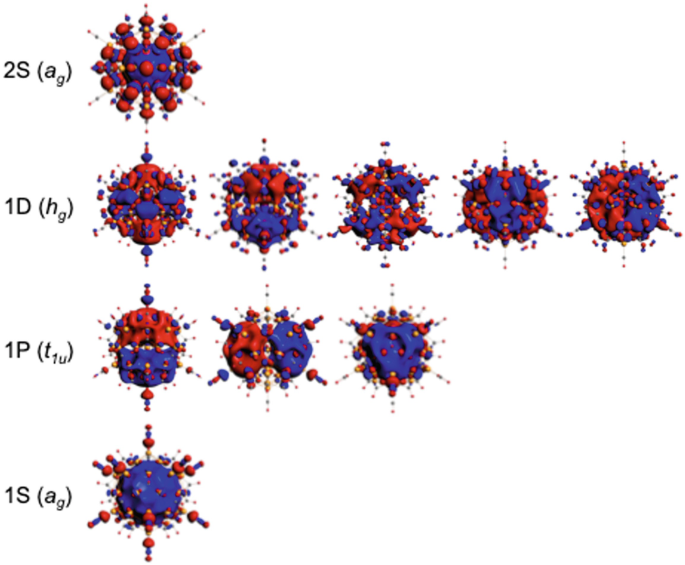
Electron Counting In Ligated High Nuclearity Late Transition Metal Clusters Springerlink

Group 10 Metal Cyanide Scaffolds In Complexes And Extended Frameworks Properties And Applications Sciencedirect

Webelements Periodic Table Silver Properties Of Free Atoms

Heteroleptic Trivalent Chromium In Coordination Chemistry Novel Building Blocks For Addressing Old Challenges In Multimetallic Luminescent Complexes Sciencedirect

Introduction To Structure Of Atom Proton Neutron Electron With Examples
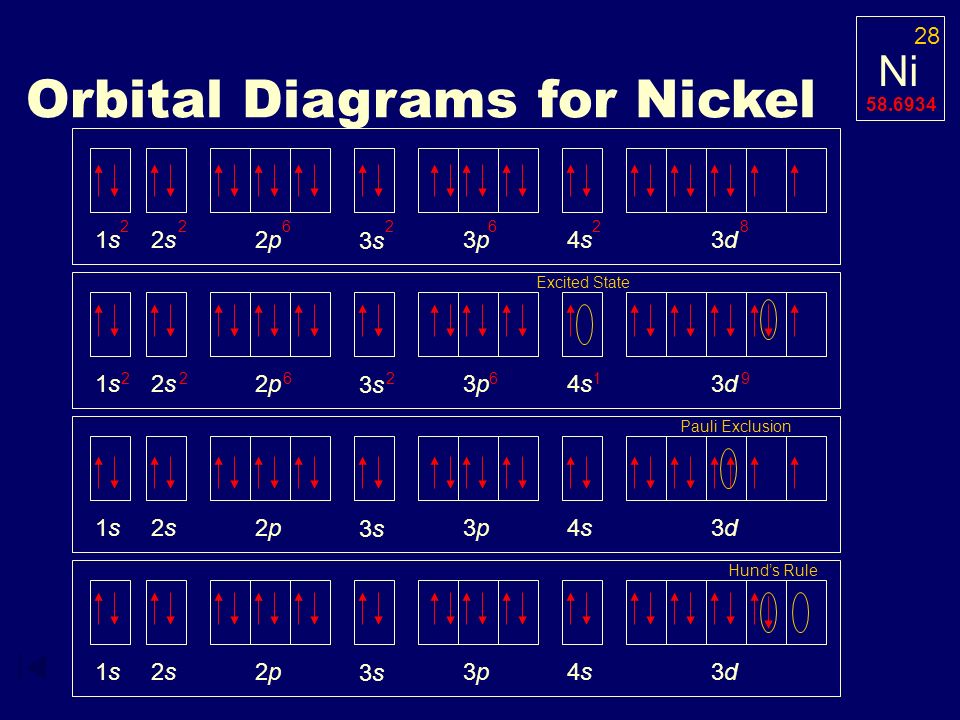
Electron Configuration Ppt Video Online Download

Webelements Periodic Table Silver Properties Of Free Atoms